


Global R&D Network
To enable our transformation, we strive to migrate adult smokers to lower risk*†, smokeless forms of tobacco and nicotine. Our focus continues to be on finding and developing new innovations and products and establishing data-based insights that evolve our innovation strategy to create products that could reduce the burden on global Public Health.
This is work undertaken by 1,750 R&D specialists across eight sites around the world. BAT’s primary scientific research centre is in Southampton, UK.
Supported by an ecosystem of R&D facilities, including Innovation and Product Centres. The Innovation Centres in Southampton, Shenzhen and Trieste collaborate on product development and innovation.
Although part of the BAT Group, Reynolds American undertakes its own research and science in support of its regulatory submissions. Visit the Reynolds Science website to find out more.
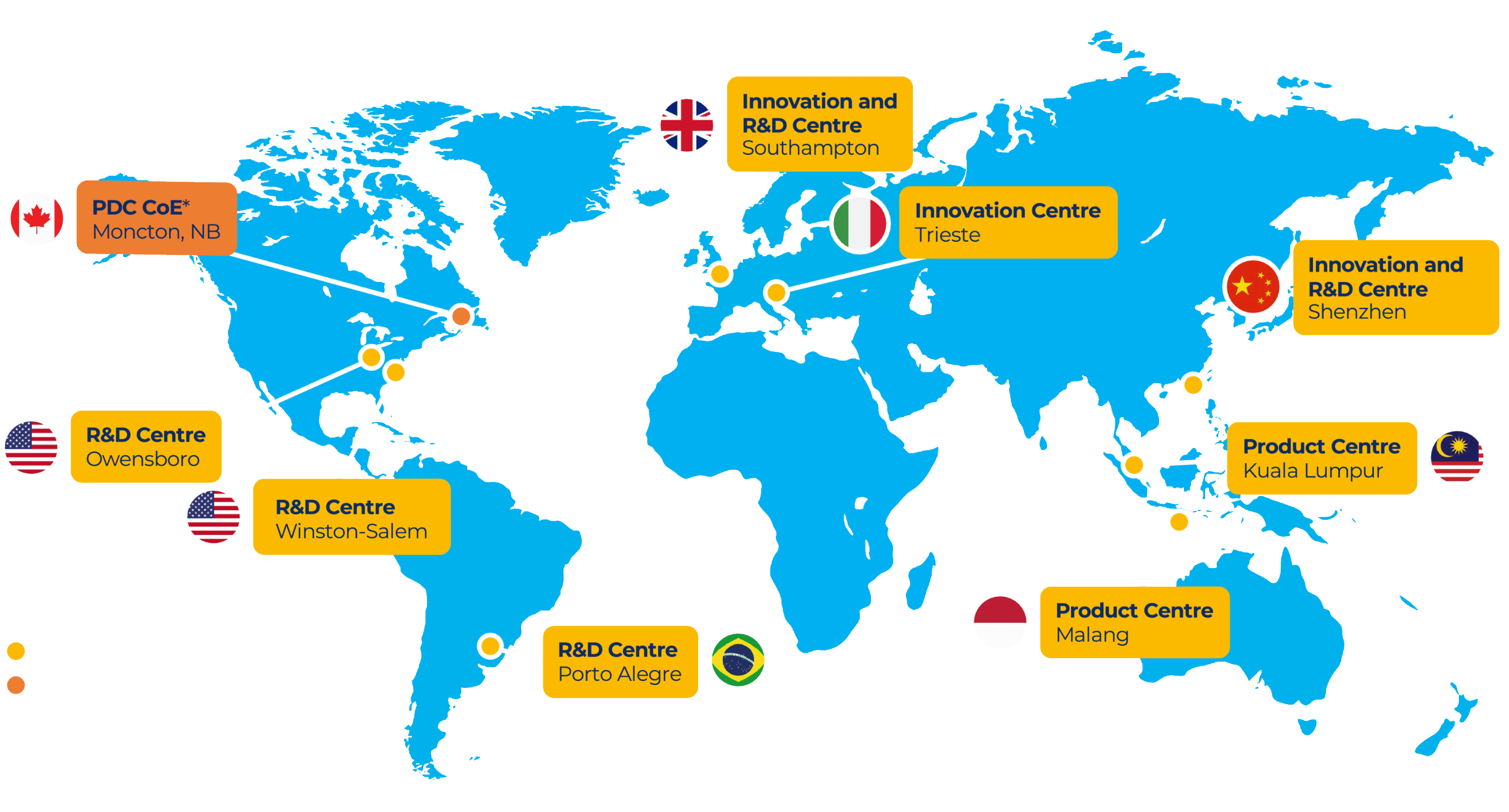
R&D Centre, Southampton, UK
Established in 1956, Southampton has been the central home of BAT’s R&D for almost 70 years. Initially a centre of excellence for chemistry, our capabilities have grown significantly over the decades with growth in biology, toxicology, consumer behaviour, clinical studies, and mechanical / electrical engineering, that have helped advance our understanding towards developing and establishing our multi-category smokeless portfolio.

Innovation Centre, Southampton, UK
We are continuing to invest in the capabilities at our Southampton R&D Centre and recently opened an Innovation Centre that includes state-of-the-art laboratories and Modern Oral nicotine pouch pilot plant. These investments into our capabilities align to our ambition for a step-change in our obsession with innovation and hit our ambitions in Building a Smokeless World.

Reynolds American R&D Centre, Winston-Salem, US
Reynolds’ Bowman Gray Technical Center (BGTC) hosts approximately 250 scientists, product, and regulatory experts trained in a variety of disciplines, including chemistry, engineering, toxicology, preclinical biology, clinical research, behavioural science, and statistics & modelling. BGTC’s analytical testing labs are ISO 17025:2017 accredited, GLP/GMP compliant, and run 128 routine analytical methods in support of Reynolds’ regulatory submissions, product deployment activities, and manufacturing operations. Additional capabilities include a 140,000 square foot pilot plant and multiple product development labs supporting US-specific development and deployment for Reynolds’ product portfolio.

Innovation Centre, Shenzhen, China
First established as a joint venture with BAT Operations, BAT R&D entered China in 2019 with a focus on establishing a technical hub for device development. Since 2019, the Shenzhen Innovation Centre has grown significantly and has a wide range of experts who have rich experiences in mechanical and electrical engineering, industrial design, device safety, compliance, validation, and certification. Our R&D presence in China enables BAT to develop strategic partnerships in a region that is home to around 90% of the world’s smokeless device suppliers.

Innovation Centre, Trieste, Italy
Opened in 2023, the Innovation Centre in Trieste demonstrates BAT’s drive to build a sustainable enterprise of the future. In addition to production lines for BAT’s smokeless products, the Centre has a pilot plant for the development of new product innovations. It operates using 100% energy from renewable sources.

R&D Centre, Cachoeirinha, Brazil
Brazil’s R&D centre integrates multiple R&D functions in a single location, creating a perfect environment for synergies and innovation. The site proudly stands as the second-largest analytical capability laboratory in South America, providing services for BAT operations around the world. It houses extensive analytical services laboratories, sensory, flavour development, prototyping hub, data management, product regulatory compliance, product and packaging development, as well as global leaf agronomy that forges tools and strategies through data science for all R&D.

Product Centre, Kuala Lumpur, Malaysia
Kuala Lumpur is a hub for tobacco product development, packaging innovations, leaf sourcing and product regulatory compliance. The team based here manage heated product deployment for markets within the APMEA region.

Product Centre, Malang, Indonesia
Indonesia’s R&D centre is a focused hub with capabilities that work in synergy, where the product development team can use the prototyping hub for smaller-scale testing of their products, as well as undertaking several analyses in the Analytical Services Laboratories. This centre is strategically positioned within our global R&D network, contributing valuable information to enhance our product portfolio and meet evolving business needs.

References
* Based on the weight of evidence and assuming a complete switch from cigarette smoking. These products are not risk-free and are addictive.
† Our vapour product Vuse (including Alto, Solo, Ciro and Vibe), and certain products, including Velo, Grizzly, Kodiak, and Camel Snus, which are sold in the U.S., are subject to FDA regulation and no reduced-risk claims will be made as to these products without agency clearance.
Leadership Team
![get_sub_field('image')['alt']](/wp-content/uploads/2024/07/headshot_james_murphy.jpg)
Dr. James Murphy
Director, Research and Science

![get_sub_field('image')['alt']](/wp-content/uploads/2025/04/Chris-Junker66.jpg)
Dr. Chris Junker
Group Head of Life Sciences

![get_sub_field('image')['alt']](/wp-content/uploads/2024/07/headshot_danielle_tower.jpg)
Danielle Tower
Group Head of Scientific & Regulatory Affairs

![get_sub_field('image')['alt']](/wp-content/uploads/2024/07/headshot_allen_griffiths.jpg)
Dr. Allen Griffiths
Group Head of Science Discovery

![get_sub_field('image')['alt']](/wp-content/uploads/2024/07/headshot_hugo_tan.jpg)
Dr. Hugo Tan
Global Medical Safety Officer

![get_sub_field('image')['alt']](/wp-content/uploads/2024/07/headshot_tim_nestor.jpg)
Tim Nestor
Executive Vice President, Research & Science, Reynolds America

