
How we test our products
We use a peer-reviewed scientific assessment framework to assess emissions, exposure and risk of our products, when compared to smoking cigarettes. As part of this, our framework builds a comprehensive set of scientific evidence and a more holistic picture of our products.
Over time, the presentation of our approach has evolved; however, the principles behind how our weight of evidence approach is used to assess our smokeless products has remained constant.
We look to understand:
How the product works;
How the product may impact an individual;
How the product may impact a population, and
What the effect of time may be.
The original presentation of our 9-step scientific risk assessment framework used the headings of Emissions, Exposure, Risk, and Harm. The evolution of this framework now refers to these events as Product, Individual, Population, and Effect Over Time. We believe these titles better explain the scientific evidence regarding our smokeless product’s effects at each assessment stage.
Spacer




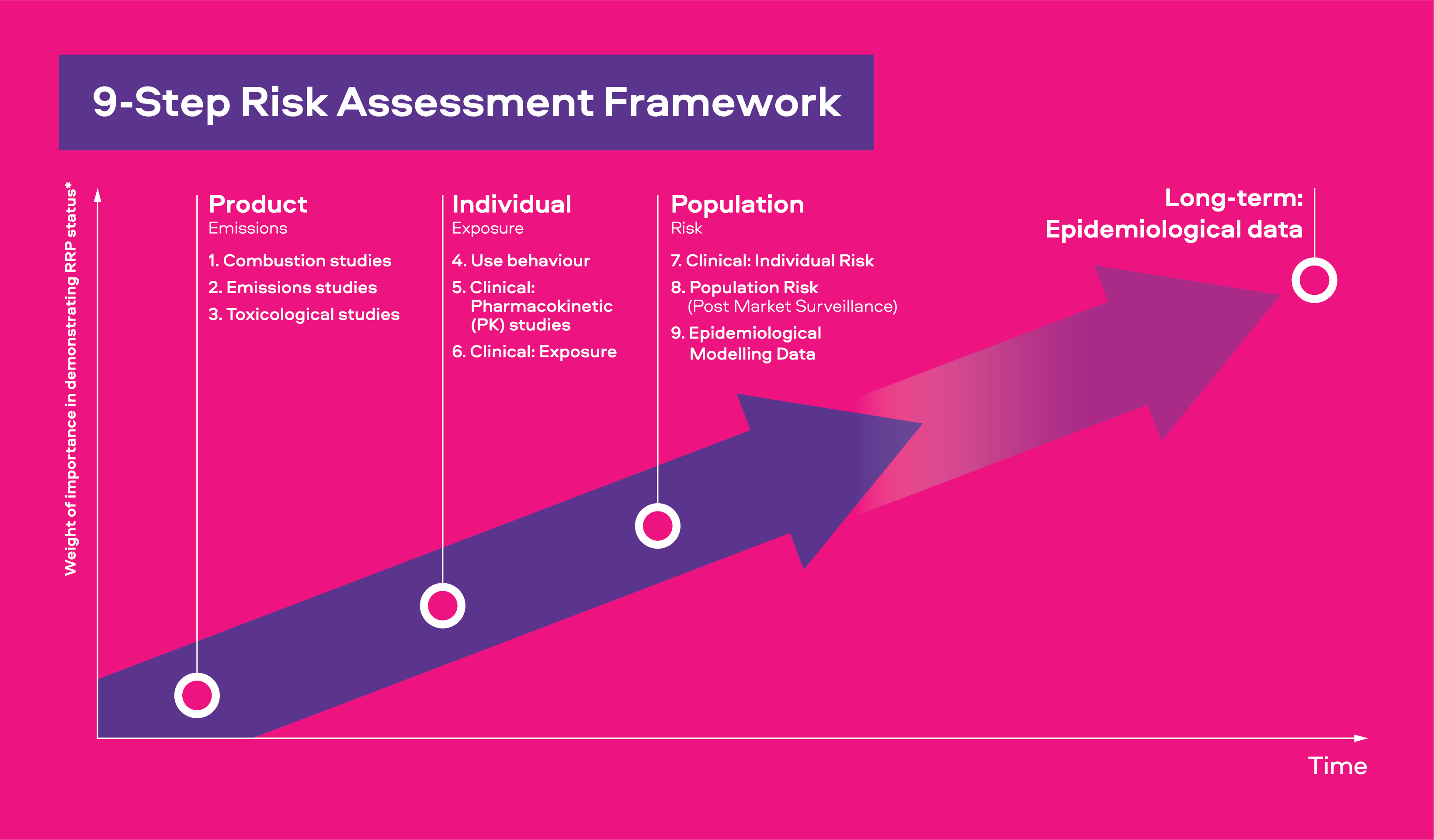
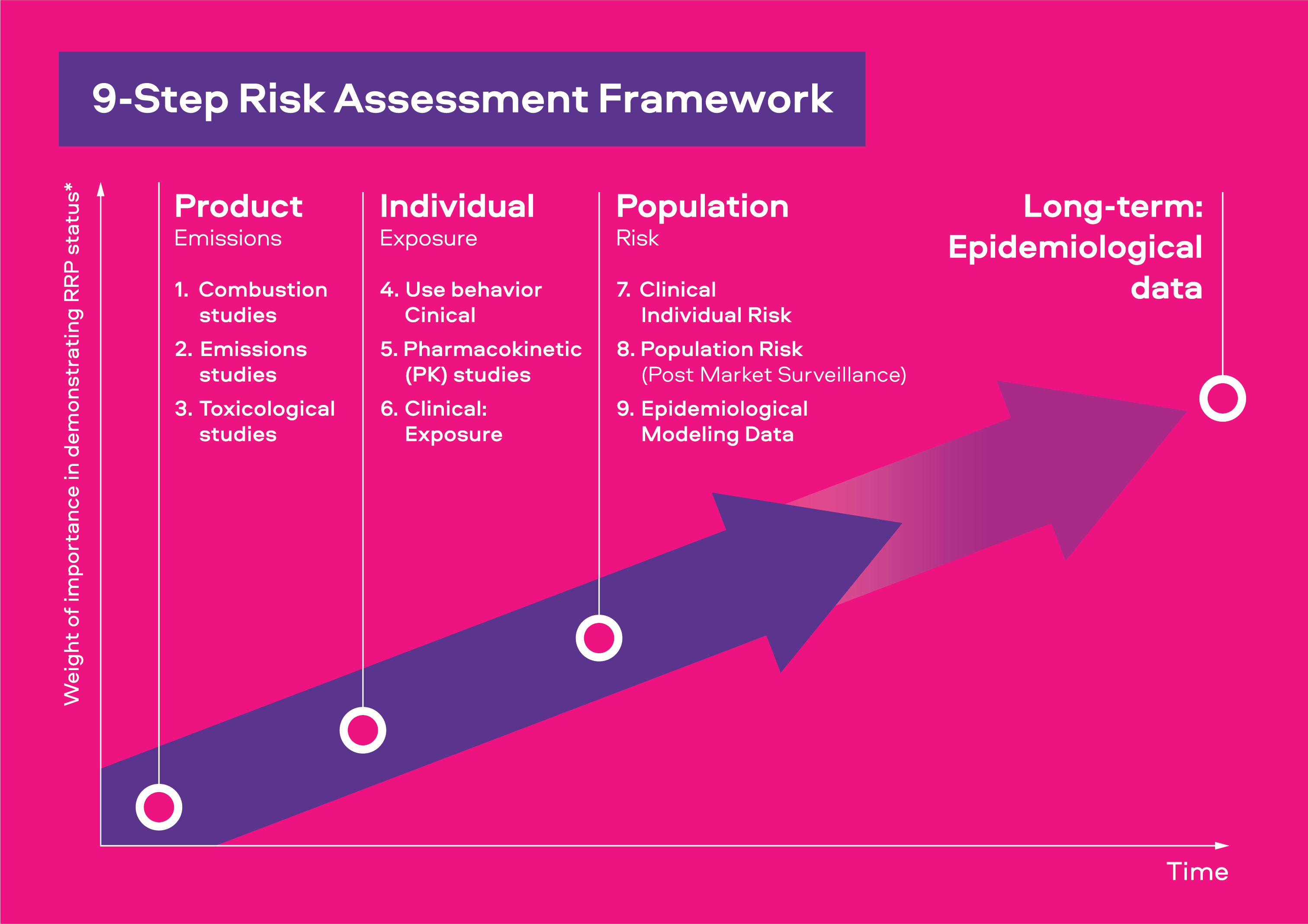
There are three main areas of research:
Product
Studies to measure the identities and levels of the chemicals released from products.

Combustion studies
It is the combustion of tobacco in cigarettes that produces the thousands of chemicals in smoke. We have established a series of analytical techniques to determine that there is no combustion in our new alternative tobacco and nicotine products.
Emission studies
In the absence of combustion, the aerosols of our Vapour and Heated Products are simpler, compared to cigarette smoke. We measure the levels of toxicants in these aerosols and compare them to those found in cigarette smoke, demonstrating significant reductions.
Oral Nicotine Pouches do not form an aerosol. We measure the toxicant levels that can be extracted from these products and compare these levels to those found in cigarette smoke. With no combustion or tobacco, these products show the lowest levels of toxicants compared to cigarette smoke.
Toxicological studies
With our new smokeless products having simpler aerosols and extractions, and lower levels of toxicants, we then determine their impact on biological cells and tissues compared to cigarette smoke. Our toxicological assessments use a variety of standardised historical and contemporary cell- or tissue-based test methods. These studies help determine whether the aerosols and extractions of our new smokeless products have reduced toxicity compared to cigarette smoke.

Individual
Once we have proven that a smokeless product can offer smokers who switch a reduction in toxicants and toxicity, we determine whether adult consumers may use the product in a manner in which negate these reductions.

Use Behaviour
We conduct clinical studies with current adult smokers to understand how consumers use the product and how often they use it. This information helps assess users’ exposure to product emissions and informs the design of our laboratory and clinical studies, which aim to mimic actual usage patterns.
Clinical: Pharmacokinetic (PK) studies
We conduct clinical studies to compare how nicotine from our smokeless products is absorbed and metabolised before, during, and after product use. We compare this data to historical cigarette data. This is important to demonstrate the effectiveness of our smokeless products to help encourage smokers to switch to a better alternative.
Clinical: Exposure
We conduct clinical studies to determine whether the reduction in toxicants in our smokeless products’ aerosols and extractions result in reductions in exposure for adult smokers who switch completely to the new product. Biomarkers that indicate exposure to certain toxicants are measured in the user’s urine and bloodstream. We compare smokers who switch to smokeless products, with smokers who continue to smoke, and smokers who quit.

Population
Once we have proven that adult consumers will use our products in a manner that does not offset their reductions in toxicant delivery compared to cigarette smoke, we look to determine what the long term and population effects could be.

Clinical: Individual Risk
We conduct longer-term clinical studies of six months-to-a-year duration. We evaluate changes in biomarkers of potential harm (BoPH). These BoPH are associated with the development of smoking-related diseases. Overall, all our smokeless products showed favourable changes in these biomarkers by smokers who switched completely.
Population Risk (Post Market Surveillance)
We monitor the natural consumption behaviour of a population over time to understand potential effects relevant to Tobacco Harm Reduction. We have evidence that demonstrates average daily consumption of our smokeless products is comparable and/or lower than smoking consumption rates.
Epidemiological Modelling Data
We have developed and validated a modelling tool to assess a wide range of scenarios that consider potential benefits and harms from changes in tobacco and nicotine product use patterns. We can model potential survival benefits in groups of smokers who completely switch to smokeless products as compared to continued smoking. We can model this data against the effects of never and former smokers adopting the new product. These types of studies model human health risks and public health outcomes over time.

Effect of Time

We know it is not possible to accelerate time; however, understanding the long-term effects of using smokeless tobacco and nicotine products is important. We are constantly assessing how we can leverage available Real-World Evidence and Data from smokers who have switched to smokeless products, long-term.
Long-term epidemiological data exists for traditional tobacco oral products (snus) that demonstrate its Tobacco Harm Reduction potential. Through our risk assessment framework, we have demonstrated that Oral Nicotine Products have reductions in toxicants, toxicity and exposure comparable and lower than snus, which would support the bridging of snus epidemiological data to the Oral Nicotine Pouch category.

References
* Based on the weight of evidence and assuming a complete switch from cigarette smoking. These products are not risk free and are addictive.
† Our vapour product Vuse (including Alto, Solo, Ciro and Vibe), and certain products, including Velo, Grizzly, Kodiak, and Camel Snus, which are sold in the U.S., are subject to FDA regulation and no reduced-risk claims will be made as to these products without agency clearance.
Publications and Conferences
Our commitment goes beyond simply conducting the science, we believe it is as important to share it with the wider scientific community, which is why we publish details of our scientific research on this website and submit the results of our research to peer-reviewed scientific journals.
Discover more

